
Jurisdiction Update November 2022
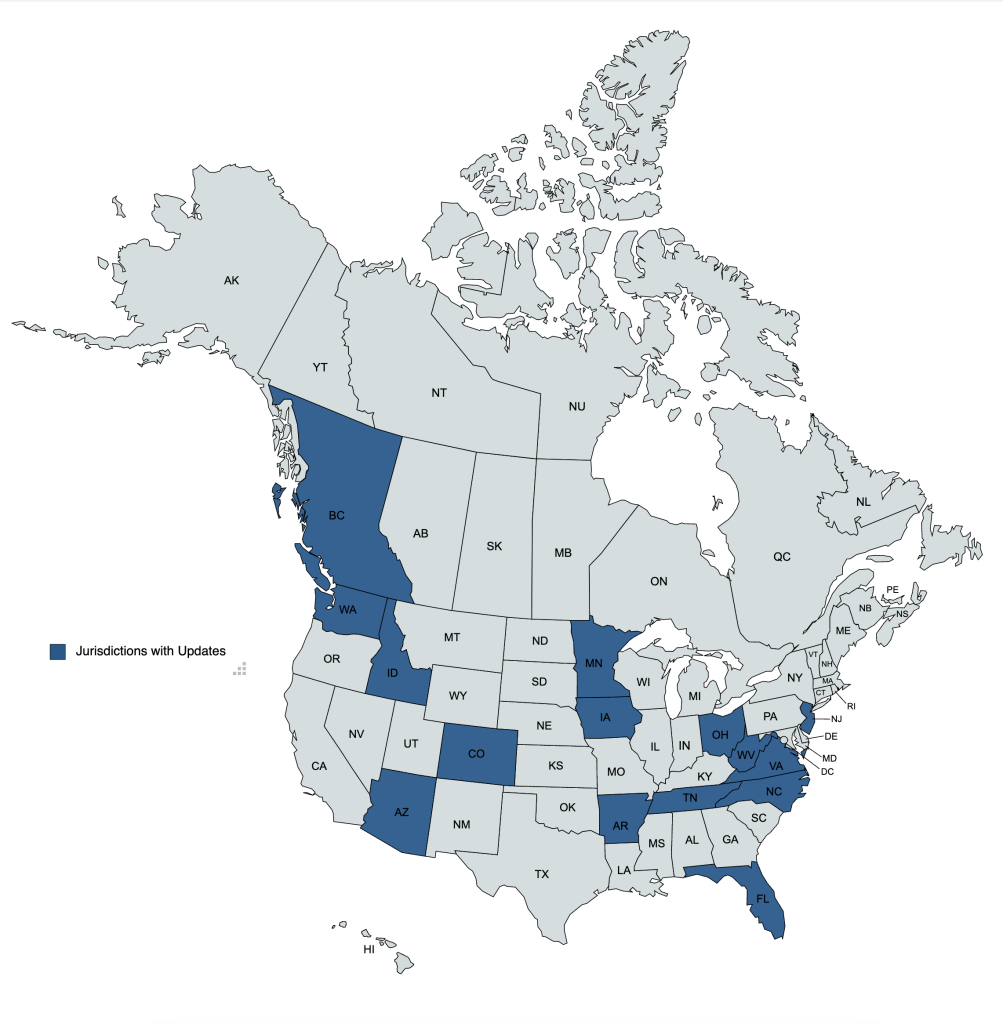

Monthly reports share short summary highlights of tracked legislative bills and rules & regulations that have seen recent activity, as well as available board and state VMA updates.
There are two report views available! The November 2022 Conventional Report sorts the same reported items by activity and topic.
Updates by Jurisdiction
Arkansas
The WV Board of Veterinary Medicine is requesting comments on the Board’s proposed Chapter 30 Law Change. The deadline to submit public comments is Wednesday, November 30, 2022.
Click here to view the law changes.
Hearing/comments end November 30, 2022
Board Summary:
10‐1 Unlawful Acts
Added language from AAVSB PAM
10‐3 Definitions
Modified General Supervision
Modified Indirect Supervision
Modified Practice of veterinary technician or “veterinary technology”
Added Person
Added Telehealth Services
Removed Veterinary Assistant
10‐5 Powers of the Board
Revised a.7 (online examinations)
10‐8 Requirements for Veterinary License
Added to the title “or veterinary telehealth practitioner registration” Removed a.1. “Be of good moral character”
Combined 2.a. and 2.b.
Removed “moral turpitude” from b.6.
Added “rational nexus” to b.7.
Modified b.8
10‐9 Scope of practice for a licensed Veterinary
Added to the title “or veterinary telehealth practitioner”
10‐10 Requirements for a registered veterinary technician
Added to the title “or veterinary technologist”
Removed a.1. “Be of good moral character”
Added “graduate of foreign veterinary technician or technology school” Removed “moral turpitude” from a.6.
Added “rational nexus” to a.7.
Added “violence, or of sexual nature” to a.8.
10‐11 Scope of practice for registered veterinary technician
Added to the title “or veterinary technologist”
Revised the scope of practice done under “general supervision”. Removed general supervision and modified what can be done under “indirect supervision to b.
10‐12 Requirements to be a certified animal euthanasia technician
Cleaned up the title to match the other titles
Added rational nexus
Added “animal abuse or neglect, violence, or of a sexual nature
10‐12 Requirements for certified animal euthanasia technicians’ program
Removed section d. US States Department of Justice character certification language
10‐15 Renewal Requirements
Removed “or biennially” from a.
Removed section b.
Added military language
Added low‐income language
10‐16 Temporary Permits for a veterinarian
Changed the title to “Emergency responder temporary permits for a veterinarian, veterinarian technician, or veterinarian, technologist
Added emergency responder language to a. and b.
10‐17 Exemptions from article.
Added emergency services
10‐18 Display of license, permit, registration, and certificate
Added “printed” to a.
Added “all of his or her business locations”
10‐19 Complaints, investigations, due process procedures, grounds for disciplinary action.
Removed Board of Pharmacy quarterly reports.
Added “rational nexus” to g.2.
Added “Engaging in acts of cruelty or abuse of animals”
10‐ 25. Exemption from licensure for professional practice for a charitable function.
Added language about exempting charitable function
The WV Board of Veterinary Medicine is requesting comments on the Board’s proposed §26-2-1 Rules change. The deadline to submit public comments is Wednesday, November 30, 2022.
Click here to view the law changes.
Hearing/comments end November 30, 2022
Removes the requirement for a complainant to include a notarized statement when making a complaint to the Board
Allows the Board to notify the licensee by any means as long as they can provide proof of delivery; Previous rules required certified mail.
British Columbia
Telemedicine
This item is meant to keep Council apprised of the evolving state of telemedicine, as the College is receiving questions from registrants. With new telemedicine platforms popping up and a variety of models being used, the CVBC needs to make registrants are aware of how to provide these services appropriately and the College is communicating with the registrant body as a whole, via our blog (1 of 2 planned entries have been posted), and also responding directly to registrant questions via email responses to questions and directions to the website’s blog and posted Guidelines. The office is seeking input from Council on any other ways we might expand the numbers of registrants we are reaching. Dr. Thomas has also reviewed and updated the Telemedicine FAQ document that was first developed in 2021, to better reflect the questions (and misunderstandings) that we have identified over the last year. Council suggested making a podcast to answer questions solicited from registrants, to be posted on the website so registrants can listen to it on their own time.
Council was informed that this is a topic that is being discussed across all the provinces and at the AAVSB, and summarized the key difference between our policy and others:
1. Some provinces and states do not allow establishing a VCPR virtually
2. Some provinces and states do not allow prescribing of controlled drugs, or it is extremely limited
3. Some provinces and states have developed a class of practice accreditation for telemedicine service providers
As with all matters, the College needs to consider the benefits and/or risks of telemedicine to the public.
Dr. Mancell advised Council that she has been exploring providing telemedicine services recently and is interested to understand the requirements. In summary: in order to provide services, a veterinarian must be a CVBC registrant and affiliated with a facility, and that the client and patient must be in BC; if the CVBC-registered practitioner is not physically in the province, they must declare that at the outset as part of the informed consent discussion. The veterinarian providing the telemedicine services is providing them through their affiliated practice facility – therefore, the Designated Registrant (+/- owner) of the facility in question must be made aware (and be supportive) that they are doing this because if there is a complaint made, the facility/DR could be involved as well.
The Accreditation Standards do identify that the standards applicable to a particular facility will reflect the declared scope of practice of that facility. PFAC will need to consider whether or not the bylaws support accreditation for just telemedicine services, and which accreditation standards would be requirements and to develop a policy document within the existing accreditation standards framework to establish telemedicine practices as a recognized limited form of accreditation and clearly outline requirements. “Limited Accreditation” allows PFAC to define the limitations of the activities of a facility so accredited – if a DR then wished to provide broader services, they would need to request a modification of the terms of their accreditation.
Direction: to pursue the development of a framework for accreditation through PFAC, including research of other jurisdictions so that that work can build upon what others have already learned, before bringing it back to Council for input and approval
Colorado
Colorado Prescription Drug Monitoring Program Legislative Update and Training Webinar Recording and Summary/FAQ Document Now Available
In August and September 2022, the Colorado Prescription Drug Monitoring Program (PDMP) hosted three webinars concerning several statutory updates to the Colorado PDMP as well as training and resources regarding registration and use of the Colorado PDMP. A recording of the August 23rd webinar, copy of the presentation slides, and a Summary and Frequently Asked Questions (FAQ) document are now available at dpo.colorado.gov/PDMP/Training.
Florida
Our Resources for Telehealth Legislation
Telehealth legislation has been voted upon in recent years and because of its potential to impact animals, we are preparing for it to reappear.
The FVMA has long supported the veterinary telehealth legislature that provides protections for the veterinarian-client-patient relationship (VCPR), and our commitment remains to nurturing opportunities that properly protect animals, their owners, and the veterinarians who support them.
Want to make an impact? Staying informed and getting involved, as well as financial assistance, helps us continue fighting on behalf of both animals and humans.
The Telehealth Coalition
Spearheaded by the American Veterinary Medical Association (AVMA), the coalition intends to collaborate across the veterinary and animal health industry to enhance and expand care by leveraging technology while safeguarding the welfare of animals and people.
The coalition’s members are essential in helping better position veterinary practices to tackle telemedicine, and are an essential part of protecting the VCPR.
LEARN MORE FROM DR. GAIL GOLAB
The FVMA is proud to be a part of the coalition alongside its many partners and associations.
Our Telemedicine Bill
Just as so many veterinarians emphasize transparency regarding their clients, we see it as our duty to be transparent with you in our fight for veterinary medicine.
We are currently working with the draft version of our own bill, where research and conversations with all affected parties has led to proposed legislation we believe can serve as equal protection for pets, owners, and veterinarians alike.
READ THE DRAFT OF OUR TELEHEALTH BILL
Our position
As members of the Telehealth Coalition, we believe veterinary telehealth, particularly telemedicine, holds great promise for improving continuity of care and strengthening the relationship between veterinarians, their clients, and their patients. We also support the recommendation of the AVMA that a VCPR should not be established via electronic means.
Idaho
24.38.01 – Proposed Rules of the State of Idaho Board of Veterinary Medicine
Docket No. 24-3801-2200F (Fee Rule)
Notice of Omnibus Rulemaking in October issue – pg. 720
This proposed rulemaking publishes the following rule chapters previously submitted to and reviewed by the Idaho Legislature under IDAPA 24, rules of the Division of Occupational and Professional Licenses / Board of Veterinary Medicine:
IDAPA 24.38
• 24.38.01, Rules of the State of Idaho Board of Veterinary Medicine.
The rules govern the licensing procedures, supervision requirements, standards of practice, inspections, and grounds for discipline of veterinarians, veterinary technicians, Committee on Humane Euthanasia members, and certified euthanasia technicians and agencies.
The proposed text of the omnibus fee docket no. 24-3801-2200f (new chapter) begins pg. 721
Iowa
Telemedicine – The Governor’s office lifted the Proclamation. The Iowa Board of Veterinary Medicine listed a notification on the Board’s website under Board News and Announcements: Effective October 1, 2022, the Iowa Board of Veterinary Medicine (IBVM) is no longer suspending the enforcement of a violation of 811 Iowa Admin. Code 12.1(2) where a veterinarian cannot establish a VCPR based solely upon a telephonic or electronic communication. The Iowa Veterinary Medical Association was asked to notify their members by placing the rescinded telemedicine information in the member newsletter.
Minnesota
Minnesota BVM Bits – Fall 2022 Newsletter
Updates on Regulation of Hemp-Derived Cannabinoid Products
Veterinarians that are asked about, recommend, or sell hemp-derived products should be familiar with the Board of Pharmacy’s Guidance on Hemp-Derived Cannabinoid Products. The full document and answers to FAQ’s can be found on the Board of Pharmacy’s website home page under Notices: https://mn.gov/boards/pharmacy/. Important points in the Guidance were summarized in the Board of Veterinary Medicine’s Spring newsletter.
In Minnesota, the same regulations apply to both human and animal cannabinoid products. With the rapid proliferation of CBD products available to animal owners, how can a veterinarian verify that the analysis of the cannabinoid amounts in a product is accurate? This is a significant challenge, as only an independent, accredited laboratory can certify that the product is compliant. This means it is not a drug (exceeds limit) and the cannabinoid content matches the amount or percentage on the label. The Office of Medical Cannabis at the MN Department of Health has a process in place to approve laboratories that perform the analysis. However, a readily accessible list of such laboratories is not yet available.
A recent investigation found that only 42% of CBD products marketed in the U.S. match the percentage of CBD advertised on products’ labeling. In edible CBD products, researchers detected the presence of lead, mercury, arsenic cadmium, phthalates, and other heavy metals in a similar percentage. Heavy metal and phthalate contamination and labeling integrity in a large sample of US commercially available cannabidiol (CBD) products – ScienceDirect.
In Minnesota, these products would be classified as misbranded or adulterated, which then reclassifies them as drugs.
Enforcement of the multiple regulations pertaining to hemp-derived cannabinoid products is increasingly challenging and currently under the purview of the Board of Pharmacy. The Board of Pharmacy strongly supports legislation to create an Office of Cannabis Management to have authority over everything related to Cannabis sativa, including CBD.
Federal Drug Administration (FDA) approval of any cannabinoid products as animal drugs is progressing but no product has yet been approved. Hence, veterinarians should ensure that clients understand that efficacy of CBD products for addressing animal health issues has yet to meet federal criteria for legal approval.
Hemp-derived products manufactured for animals may be consumed by humans, including children. Accordingly, prepackaged CBD products and any products sold by veterinarians are required to be child resistant. No images of persons, animals, or fruit should be used on the products or advertising. Veterinarians that are asked about hemp-derived products or choose to sell them should be familiar with this guidance.
Questions pertaining to this Guidance should be directed to the Board of Pharmacy via email:
PHA.inspector.HLB@state.mn.us
New Jersey
NJ S3327: Insurance policies must disclose if it excludes coverage for certain issues, including a waiting period, preexisting conditions, etc., requires specific language, must provide 15 days to decline (specific language), must disclose benefit schedule, usual and customary fees limitations, if a vet exam is required, provide disclosure and policy provisions, more specific language requirements, waiting periods cannot exceed 30 days, cannot require an exam for renewal, cannot be tied to a wellness exam, cannot market them together, specific language on wellness programs, insurers must be trained.
Regulatory Affairs Discussed at NJ State Board of Veterinary Medical Examiners October Meeting
N.J.S.A. 45:1-15.9 – Issuance of license, certification, registration
The Board shall not renew a license if there is information that indicates that a person has been convicted of certain crimes. This is retroactive and pursuant to statute that took effect January 2022.
Taken as informational.
Opioid Continuing Education and Rowan/Division Courses
There are no courses accepted by the NJ Board of Medical Examiners that meet the requirement to obtain at least one hour every renewal cycle on opioid drugs. Rowan University offers this course which is recognized by the AMA. The NJ board of Veterinary Medical Examiners does not recognize AMA courses. The question posed to the Board: Should the Board recognize a waiver for licensees to take the course offered by Rowan University? The waiver would be only for this course at Rowan University, which is offered online.
A motion was made to provide a waiver for the online opioid drugs course offered by Rowan University. Motion seconded. Motion passed unanimously.
The board will work through the NJVMA to contact the Dean of Rowan University to see if the veterinary school can obtain RACE approval for specific CDS course at Rowan University for opioid evaluation.
Legible Records
Currently, when records provided to the Board are illegible, the Board requests a transcript. The Board expressed concern that another practitioner/owner should be able to read the record, as well.
A motion was made to change the Board’s regulations to mirror the language of the Board of Acupuncture regulation regarding legible records. Motion passed unanimously.
A revised legible records regulation proposal will be provided to the Board at the next meeting.
Senate Bill NJ S3137 Posting of Fees
It was motioned and seconded to direct the board’s Regulatory Analyst to provide the Board’s comment when asked, that the Board opposes this bill on the basis that no other profession has such a regulation.
Motion approved unanimously.
Legislative Update
Fall 2022
NJVMA Currently Monitoring 32 Bills in the State
An active advocacy voice for veterinarians is essential to provide a productive and safe environment for veterinary practice in NJ. NJVMA provides that voice through its legislative committee and retention of a legislative agent to interface with the NJ legislature and administration.
The NJVMA Legislative Committee is comprised of the following members: Dr. Mike Yurkus, Chair; Dr. Barry Adler; Patricia Smillie-Scavelli, Esq.; Dr. Charlotte Lacroix; Dr. Robert Gordon; Dr. Todd Wolf; Dr. Joe Chiosi; Dr. Josh Portner; Dale Florio, of Princeton Public Affairs Group; Richard Alampi, NJVMA Legislative Consultant; and Phil Russo, Executive Director.
NJVMA continues to advocate on behalf of the veterinary profession in NJ by supporting legislation that advances the profession and opposing legislation that would hinder the practice of veterinary medicine.
The number of bills that impact the profession and animals is substantial. The NJVMA legislative committee and our legislative agent, Dale Florio, are currently monitoring 32 bills. Following are some of the key issues in the legislature.
- Animal Advocate- S2868 would provide for an advocate to be appointed in criminal cases concerning welfare or care of animals. NJVMA is part of a broad coalition opposing the bill, due to concerns with language in the bill that could change the legal status of animals to provide them with personhood.
- Facility Inspection - NJVMA successfully opposed A317 which would have required an annual inspection of veterinary facilities.
- Veterinary Technicians – The NJVMA legislative committee recommended opposing S4168, which would require licensure for veterinary technicians. The committee’s position is that there is a critical shortage of credentialed (and even uncredentialed) technicians that is not likely to be alleviated in the near future. This bill, if enacted into law, would put veterinarian employers in a dilemma, as they would be forced to decide if they were to allow unlicensed technicians to do procedures, thereby risking SBVME sanctions, or to not proceed with treatment. With the steady increase in consumer complaints against veterinarians, this would create new opportunities for litigious or unhappy clients.
- Loan Redemption - NJVMA is working with Sen. Stanfield on a bill that would provide inducements in the form of loan redemptions to attract food animal and equine veterinarians to underserved areas in NJ. In these areas, there is a critical shortage of practitioners.
North Carolina
North Carolina Veterinary Medical Board Regulatory Bulletin
Fall 2022 – Vol. 6-3
Inside this issue: Verifying Veterinary Licenses (It is essential for employers to verify the license/registration of new hires) | NCVMB License and Registration Renewal Opens November 1, 2022 | Continuing Education Requirements | Emergency Veterinary Regulations in the NC Veterinary Practice Act | DEA Requirement – Employee Screening
Ohio
OH HB509: The Senate Workforce and Higher Education held a second hearing 11/16/22. The bill requires all occupational licensing boards to issue a report within six months of the bill’s effective date that addresses the following: the fee structure for each occupational license issued by the board, whether it can more competitively align with the surrounding states, and whether it serves as a financial barrier to licensure. Additionally, the report must also address whether the board’s process for issuing occupational licenses could be improved by using the eLicense system maintained by the Department of Administrative Services (DAS), and if so, the board must begin using that system.
The bill sets pharmacist continuing education at 30 hours every two years, instead of having continuing education requirements set by the Pharmacy Board in rules. This change will have a negligible impact on the Board’s operations.
…
(b) pursuant to division (e) of section 101.62 of the revised code, the following occupational licensing boards are hereby renewed and, subject to the revisions prescribed by this act, the statutes creating, empowering, governing, and regulating those boards are continued: (18) the veterinary medical licensing board created under section 4741.02 of the revised code; ... (c) the occupational licensing boards listed in this section shall be triggered to expire under division (b) of section 101.62 of the revised code at the end of the thirty- first day of December of the sixth year following enactment of this section.
Tennessee
The Tennessee Board of Veterinary Medical Examiners posted a list of Legislative Updates 2022
Non-Health Related Legislative Activity of Note
- The legislature addressed legislative and Congressional redistricting.
- The “Truth in Sentencing” Act made mandatory sentences for certain criminal offenses.
- The “Tennessee Investment in Student Achievement (TISA) Act” reformed the school funding approach.
Highlights and Noteworthy Health-Related Legislation
- The Department had two successful legislative initiatives that became law relating to local county health departments and the Controlled Substance Monitoring Database, respectively.
- Healthcare providers can continue to utilize telehealth and receive reimbursement for telehealth services.
- The Board of Pharmacy and the Board of Nursing will now hire and fire the Executive Director of the Board.
- A registry within the Tennessee Commission on Aging and Disability was created to combat the operation of unlicensed facilities.
The complete text of the following Public Acts is available on the following website: https://www.sos.tn.gov/division-publications/acts-and-resolutions
Virginia
VA HB1382: It is unlawful to perform a declawing procedure on a cat, except that a person engaged in the practice of veterinary medicine may perform such a procedure if it is necessary for a therapeutic purpose. No reporting requirement for veterinarians.
West Virginia
The WV Board of Veterinary Medicine is requesting comments on the Board’s proposed Chapter 30 Law Change. The deadline to submit public comments is Wednesday, November 30, 2022.
Click here to view the law changes.
Hearing/comments end November 30, 2022
Board Summary:
10‐1 Unlawful Acts
Added language from AAVSB PAM
10‐3 Definitions
Modified General Supervision
Modified Indirect Supervision
Modified Practice of veterinary technician or “veterinary technology”
Added Person
Added Telehealth Services
Removed Veterinary Assistant
10‐5 Powers of the Board
Revised a.7 (online examinations)
10‐8 Requirements for Veterinary License
Added to the title “or veterinary telehealth practitioner registration” Removed a.1. “Be of good moral character”
Combined 2.a. and 2.b.
Removed “moral turpitude” from b.6.
Added “rational nexus” to b.7.
Modified b.8
10‐9 Scope of practice for a licensed Veterinary
Added to the title “or veterinary telehealth practitioner”
10‐10 Requirements for a registered veterinary technician
Added to the title “or veterinary technologist”
Removed a.1. “Be of good moral character”
Added “graduate of foreign veterinary technician or technology school” Removed “moral turpitude” from a.6.
Added “rational nexus” to a.7.
Added “violence, or of sexual nature” to a.8.
10‐11 Scope of practice for registered veterinary technician
Added to the title “or veterinary technologist”
Revised the scope of practice done under “general supervision”. Removed general supervision and modified what can be done under “indirect supervision to b.
10‐12 Requirements to be a certified animal euthanasia technician
Cleaned up the title to match the other titles
Added rational nexus
Added “animal abuse or neglect, violence, or of a sexual nature
10‐12 Requirements for certified animal euthanasia technicians’ program
Removed section d. US States Department of Justice character certification language
10‐15 Renewal Requirements
Removed “or biennially” from a.
Removed section b.
Added military language
Added low‐income language
10‐16 Temporary Permits for a veterinarian
Changed the title to “Emergency responder temporary permits for a veterinarian, veterinarian technician, or veterinarian, technologist
Added emergency responder language to a. and b.
10‐17 Exemptions from article.
Added emergency services
10‐18 Display of license, permit, registration, and certificate
Added “printed” to a.
Added “all of his or her business locations”
10‐19 Complaints, investigations, due process procedures, grounds for disciplinary action.
Removed Board of Pharmacy quarterly reports.
Added “rational nexus” to g.2.
Added “Engaging in acts of cruelty or abuse of animals”
10‐ 25. Exemption from licensure for professional practice for a charitable function.
Added language about exempting charitable function
The WV Board of Veterinary Medicine is requesting comments on the Board’s proposed §26-2-1 Rules change. The deadline to submit public comments is Wednesday, November 30, 2022.
Click here to view the law changes.
Hearing/comments end November 30, 2022
Removes the requirement for a complainant to include a notarized statement when making a complaint to the Board
Allows the Board to notify the licensee by any means as long as they can provide proof of delivery; Previous rules required certified mail.
West Virginia Board of Veterinary Medicine News
The FDA published Guidance for Industry (GFI) #263 in June 2021 which outlined a plan for transitioning certain over-the-counter (OTC) antimicrobial products for animals to prescription (Rx) status. As outlined in FDA’s Guidance for Industry (GFI) #263, we expect approved animal drug products containing medically important antimicrobials that are currently available as OTC products to transition to Rx status beginning in June 2023. To help prepare for this transition, FDA/CVM is conducting outreach to increase awareness about this expected change. Outreach efforts so far have included disseminating information in September to some target audiences (TX, AZ, OR) through radio and social media.
CVM has also developed some written materials that we hope will be helpful. Below are links for species-specific fact sheets – these include information on products/indications that will be transitioning as part of GFI 263 and information on how interested animal owners can locate a vet. Also linked below are a brochure and poster which detail the importance of veterinary involvement as products transition to Rx.
Antibiotic Stewardship in Veterinary Medicine Brochure – https://www.fda.gov/media/162067/download
Antibiotic Stewardship Poster – https://www.fda.gov/media/162075/download
Antibiotic Stewardship in Beef and Dairy Cattle - https://www.fda.gov/media/162069/download
Antibiotic Stewardship in Poultry – https://www.fda.gov/media/162071/download
Antibiotic Stewardship in Sheep and Goats – https://www.fda.gov/media/162073/download
In addition, CVM has posted a list of affected applications on its website as well as a Farmer and Rancher Q&A.
West Virginia Board of Veterinary Medicine News
The FDA published Guidance for Industry (GFI) #263 in June 2021 which outlined a plan for transitioning certain over-the-counter (OTC) antimicrobial products for animals to prescription (Rx) status. As outlined in FDA’s Guidance for Industry (GFI) #263, we expect approved animal drug products containing medically important antimicrobials that are currently available as OTC products to transition to Rx status beginning in June 2023. To help prepare for this transition, FDA/CVM is conducting outreach to increase awareness about this expected change. Outreach efforts so far have included disseminating information in September to some target audiences (TX, AZ, OR) through radio and social media.
CVM has also developed some written materials that we hope will be helpful. Below are links for species-specific fact sheets – these include information on products/indications that will be transitioning as part of GFI 263 and information on how interested animal owners can locate a vet. Also linked below are a brochure and poster which detail the importance of veterinary involvement as products transition to Rx.
Antibiotic Stewardship in Veterinary Medicine Brochure – https://www.fda.gov/media/162067/download
Antibiotic Stewardship Poster – https://www.fda.gov/media/162075/download
Antibiotic Stewardship in Beef and Dairy Cattle - https://www.fda.gov/media/162069/download
Antibiotic Stewardship in Poultry – https://www.fda.gov/media/162071/download
Antibiotic Stewardship in Sheep and Goats – https://www.fda.gov/media/162073/download
In addition, CVM has posted a list of affected applications on its website as well as a Farmer and Rancher Q&A.